Content brought to you by Motor Age. To subscribe click here.
What you will learn:
• Battery technology has evolved and so has the charging equipment and strategies
• Battery electrical loads are a lot higher now, compared to years ago
• Battery state of health and state of charge ultimately determine its state of function
Decades ago, the automobile’s battery simply provided a voltage source to operate an ignition system, some lighting, and a starter motor. The demands of the electrical system were minimal compared to today’s standards. The intricacies of today’s automobiles yield a need for newer battery technologies. With these newer technologies comes a need for more accurate testing, a safer way of charging, and a requirement to alert the hosting vehicle of a battery replacement.
The batteries of yesteryear
In the early 1900s, some vehicles were first recognized as using a wet-cell type battery known as a flooded lead-acid battery, to provide electrical current for the engine’s starter motor. The batteries of the recent past (yet, still present in many of today’s automobiles) are similar in construction and the technology hasn’t changed much (Figure 1). batteries use a chemical reaction to generate electrical energy. Six lead plates (or cells) were arranged within the battery housing and connected in series, electrically. As the name of the battery design suggests, the lead plates were submerged in sulfuric acid (otherwise known as electrolyte).
In the past, “maintenance” meant maintaining the electrolyte level, as the electrolyte tended to evaporate over time. A lack of electrolyte leads to excessive heat build-up. This action, or even a really deep discharge, did significant harm to the useful life of the battery. During these conditions, lead sulfate would grow on the surface area of these lead plates and create what we call a “voltage drop,” (a process known as sulfation). A battery may be at 100 percent state of charge, yet source voltage would drop significantly under an electrical load (like during starter motor operation).
Regarding the lead-acid battery, its operation depends on a few aspects that can be evaluated through testing. For one, I will refer to it as capacitance. Otherwise stated, a battery’s ability to store energy. You can think of this as the size of a fuel tank. A large fuel tank has more storage capacity than a smaller tank. A battery with more capacitance can store more electrons than one with less capacitance.
Another aspect to be evaluated is one from the perspective of internal resistance. The internal resistance influences electrical current flow. The greater the resistance, the less current will flow, and this will affect the operation of both the battery output as well as its ability to take on energy (charging and discharging). This information is inferred by way of a conductance test. This test involves measuring voltage and a minimal current, to infer the resistive values of the battery. One more perspective is self-discharge. This point of view helps infer mechanical integrity. As a battery begins to fail, its ability to maintain its charge lessens.
When it comes to testing these batteries, a handful of test procedures have been at our disposal as automotive technicians:
- Specific-gravity testing (using a hydrometer or refractometer)
- Electrical load testing (carbon pile testing)
- Capacitance testing (specialized hand-held device)
- Source voltage testing (using a DVOM)
- Internal resistance (specialized hand-held device)
- Conductance testing (specialized hand-held device)
We capitalized on these tests, mainly because of how easy they were to implement. They infer battery health. One of the most immediately telling tests, the electrical load test, is also one of my favorites. Typically, the test involves monitoring the battery’s ability to maintain above a minimum voltage level while under a measurable electrical load. This frequently involved using a carbon-pile load found within many testers out there in the market. One that stands out in my mind was the VAT-40 or VAT-45 (Figure 2). This is a very reliable way to evaluate a battery as it is a true load, and mimics what the battery will undergo in a real-life situation. It will very easily flush a fault to the surface. Think of it as a stress test. The downside is that these testers were quite expensive and required a tech to walk across the shop to fetch the tool, as shops typically only owned one of them. Although reliable, it's not very efficient, if you ask me.
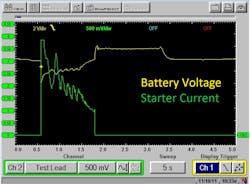
A similar alternative test is to allow the vehicle’s starter to be the load (talk about a “real-life situation”). A technician can use either a DVOM (with a MIN/MAX voltage capture function) or even a multi-trace lab scope, to monitor voltage and current simultaneously while the starter loads the electrical system, during cranking (Figure 3). Of course, the scope was very expensive, too. However, it can be used for many other situations, making its return on investment quite fruitful, and therefore an easier expense to justify.
AGM batteries and the technologies they serve
Nowadays, a more modern flooded lead-acid battery is sealed and doesn’t require electrolyte replenishment. The technology has gotten a lot better, too. A valve-regulated lead-acid battery (otherwise known as an absorbent glass mat, or AGM) is one that still relies on the chemical reaction between the lead plates and the electrolyte to take place. However, rather than flooding the battery with electrolyte, the lead plates are simply wrapped in the absorbent glass mat, and the lead plate is held against the electrolyte, rather than swimming it. This accomplishes the same chemical reaction without the need for a bath of the electrolyte.
One obvious benefit of not needing that abundance of electrolyte makes the battery ideal for vehicles like motorcycles (which spend much of their time on the road, off-kilter). Another less-obvious benefit is the fact that these batteries tend to tolerate a “deep-cycle” without any major degradation of useful life.
AGM batteries have more electrical capacity-to-weight ratio, meaning you get “more bang for your buck,” and the battery weighs less. With emissions standards getting more stringent each year, the goal of achieving maximum power delivery from the engine, with fewer tailpipe emissions and even great gas mileage, is not an easy one. Shedding every ounce of weight is of interest to any automobile manufacturer.
However, more of a concern is with the evolution of vehicle technology. Systems like “stop/start” are definitely an emissions control dream-come-true. The catalytic converter allowed for great control over dangerous pollutants like hydrocarbons, carbon monoxide, and oxides of nitrogen (HCs, CO, and NOx). However, carbon-dioxide, although naturally occurring, is considered a greenhouse gas and to reduce it means shutting off the engines. These frequent heavy loads, to be placed on the lead-acid batteries of years past would end their lives swiftly. Again, the AMG batteries are relied upon heavily, for support of the stop/start systems, because of their deep-cycle capability and smaller size.
Some other technologies to consider are the infotainment systems and others which place a significant load on the batteries, especially during key-off system operation. The deep-cycle capabilities allow the AGMs to maintain an adequate charge during these long periods of key-off parasitic loads. Some significant differences between the AGM battery and the flooded lead-acid battery were:
- Charging strategies
- Testing procedures
AGMs differ from flooded lead-acid batteries in that the charging procedures (performed by the technician) and charging system strategies (performed by the vehicle charging system) are significantly different. AGMs are much more sensitive to overcharging and can be easily damaged as a result. Specialized battery chargers must be used to alter the charge rate during a charging cycle.
The vehicle’s onboard charging system must function similarly. The ECUs that are responsible for operating the charging system monitor for voltage, temperature, and current. The system closely watches characteristics like state of charge (SOC) and state of health (SOH) to infer the state of function (SOF).
SOH is similar to the size of a gas tank (as mentioned earlier), whereas SOC is similar to how full the gas tank is. That leaves what really matters, SOF, which will basically state how far that full gas tank will carry you. I’m trying to state that how we go about testing these newer technology batteries is different, and accuracy is crucial to longevity.
What used to be a simple procedure, replacing a battery, is more complicated now. The use of a capable scan tool is a must! Post-replacement procedures exist to alert the ECU of the new battery in place. The charging system strategy and charge rate change over time, and not performing this post-replacement procedure will allow the alternator to cook the new battery and destroy it in a relatively short amount of time.
New technology breeds new tools
How we go about testing these batteries requires some different tooling to maintain accuracy and ultimately happy customers. These tools are designed for testing not only the AGM batteries but also the special charging system’s proper operation. Many of these tools on the market today are only a few hundred dollars and are a lot more capable than the tools of old (which cost thousands and are not designed for AGM battery testing). These newer tools are hand-held, which is a lot more efficient to use than the older, larger testers. Many of these tools have nifty features, like a built-in printer for a customer report or to keep shop records (Figure 4).
Some of these tools are extremely innovative and cost-effective. For instance, the Autel MaxiBAS BT608 applies an advanced exclusive battery analysis algorithm called adaptive conductance (Figure 5). Adaptive conductance produces a more accurate examination of the battery's cold-cranking ability and reserve capacity, which is vital to determining a battery's true health. This is exactly what the vehicles rely on to start the engine as well as support systems that operate during key-off (no alternator replenishment). It supports the scan tool-controlled feature of registering the new battery to the vehicle so the need for an additional stand-alone scan tool is eliminated, in this case.
Technology is a great thing if we choose to capitalize on it. The return on investment is what truly counts, so long as the tool does what it’s supposed to do. Being able to test a battery (with the most accurate algorithms), evaluate a charging system, print copies of the test results, and register a new battery, all with a single handheld, wireless device is simply amazing to me. Yes, technology is wonderful, especially in the automotive diagnostic/repair and service industry.
About the Author
Brandon Steckler
Technical Editor | Motor Age
Brandon began his career in Northampton County Community College in Bethlehem, Pennsylvania, where he was a student of GM’s Automotive Service Educational program. In 2001, he graduated top of his class and earned the GM Leadership award for his efforts. He later began working as a technician at a Saturn dealership in Reading, Pennsylvania, where he quickly attained Master Technician status. He later transitioned to working with Hondas, where he aggressively worked to attain another Master Technician status.
Always having a passion for a full understanding of system/component functionality, he rapidly earned a reputation for deciphering strange failures at an efficient pace and became known as an information specialist among the staff and peers at the dealership. In search of new challenges, he transitioned away from the dealership and to the independent world, where he specialized in diagnostics and driveability.
Today, he is an instructor with both Carquest Technical Institute and Worldpac Training Institute. Along with beta testing for Automotive Test Solutions, he develops curriculum/submits case studies for educational purposes. Through Steckler Automotive Technical Services, LLC., Brandon also provides telephone and live technical support, as well as private training, for technicians all across the world.
Brandon holds ASE certifications A1-A9 as well as C1 (Service Consultant). He is certified as an Advanced Level Specialist in L1 (Advanced Engine Performance), L2 (Advanced Diesel Engine Performance), L3 (Hybrid/EV Specialist), L4 (ADAS) and xEV-Level 2 (Technician electrical safety).
He contributes weekly to Facebook automotive chat groups, has authored several books and classes, and truly enjoys traveling across the globe to help other technicians attain a level of understanding that will serve them well throughout their careers.